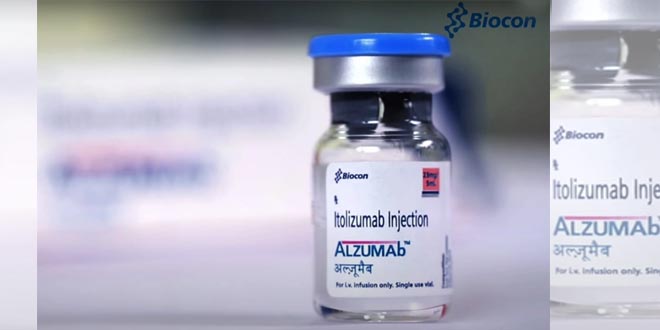
Amritsar, NFAPost: Biocon Ltd’s Itolizumab, a drug used to cure skin ailment psoriasis, has been given approval by the Drug Controller General of India (DGCI) for “restricted emergency use” to treat Covid-19 patients.
The Bengaluru-based biopharmaceutical company today said that DGCI had given approval for emergency use of the drug on Covid-19 patients with moderate to severe acute respiratory distress on the basis of its clinical trials on only 30 patients across four centres.
The company will launch the biologic drug at a price of around 8,000 rupees per vial, it said.
The cost per vial is 7,950 rupees, and as most patients need four vials, the cost of the therapy will be around 32,000 rupees, Biocon Executive Chairperson Kiran Mazumdar-Shaw said in a virtual press conference.
Itolizumab is the first novel biologic therapy to be approved anywhere in the world for treating patients with moderate to severe complications due to Covid-19, the company said.
The company has been manufacturing and marketing Itolizumab drug under the brand name ALZUMAb since 2013 for treatment of patients with moderate to severe chronic plaque psoriasis, the Ministry of Health had said in a statement on Saturday.
“This indigenous drug has now been repurposed for coronavirus,” the health ministry said.
Biocon has claimed that the clinical trials reduced mortality, improved oxygen levels, and reduced hyper-inflammation in the body. It also claimed that doctors and investigators in many parts of India have used ALZUMAb to save patients and over 150 patients have recovered in Maharashtra, Gujarat, and Delhi.
The trials had begun in May and were carried out at Lok Nayak Jai Prakash Narayan Hospital and All India Institute of Medical Sciences in Delhi, and at King Edward Memorial (KEM) Hospital and BYL Nair Hospital in Mumbai.
Biocon will now look at ramping up capacity to manufacture more vials of Itolizumab in view of the pandemic, Mazumdar-Shaw said.
“We have the manufacturing capacity and the supply and distribution network in place, however post this approval we are looking to ramp up production capacity to meet the expected surge in demand. Our aim is to reach a larger number of patients across the country,” she said.
The company will manufacture and formulate the drug as an intravenous injection at its bio-manufacturing facility at Biocon Park in Bengaluru.
Mazumdar-Shaw said that the company will be supplying the drug to hospitals as per protocol against a medical prescription and patient consent form.
“Currently, there is a huge demand and Biocon wants to ensure Itolizumab first reaches those patients who need them the most,” she added.
The Biocon chief said that even if a vaccine is developed by the end of this year or early next year, there is no guarantee that there will not be reinfection, there is no guarantee that it will work the way it is expected it to work, so we must be in a state of preparedness.
“Until the vaccine comes, we certainly need life saving drugs. I think what we are doing across the world is to see how we can either repurpose drugs or develop new drugs to treat this pandemic,” she said.