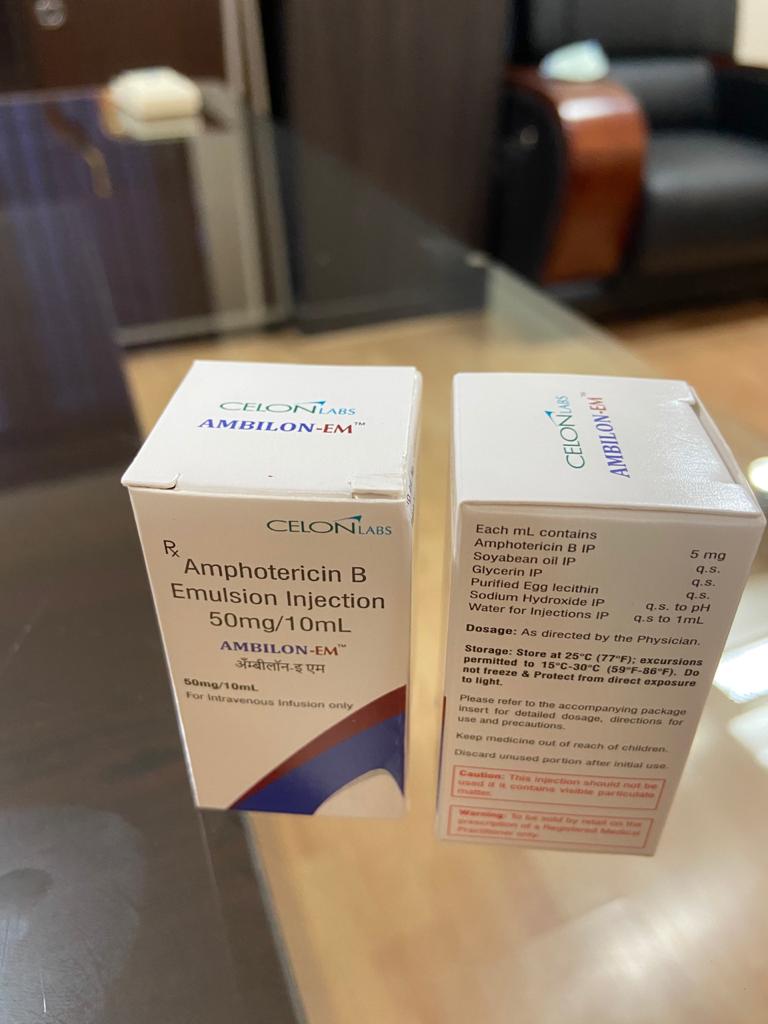
Hyderabad, NFAPost: Hyderabad-based specialty biopharmaceutical manufacturer Celon Laboratories announced the launch of an alternative drug – Amphotericin B Emulsion, for the treatment of Mucormycosis, commonly known as Black Fungus.
The company informed that it has today released the drug in the market. The drug would initially be supplied in the State of Telangana through its distributor, Jaya Surgicals.
Celon would soon be supplying the drug to the other States once sufficient stocks are supplied to the Telangana patients. The release of the drug in the market provides much-needed respite to patients reeling under the severe impact of the second wave of COVID-19 and the resultant Black Fungus.
Exhaustively developed by Celon’s in-house R&D team in a record three weeks, the emulsion-based formulation will provide leading hospitals and COVID-19 treatment centers with increased resources to treat the Black Fungus.
Celon will manufacture 10,000 vials per day, which will ensure relief for 6,000 patients monthly. The new drug has ingredients comprising of Amphotericin API, soyabean oil, egg lecithin and glycerin and is priced Rs 4250/- per vial.
Celon Laboratories Private Limited engages in the manufacturing, marketing, and distribution of specialty biopharmaceutical products specific to oncology and critical care segments. Additionally, Celon manufactures special technology-based products developed in-house, including depots, nano-particulate, and other liposomal formulations.
The company employs 600 people and commercializes its products within India as well as over 40 additional markets across the globe. Celon is owned by the London-based KELIX bio, which is backed by CDC Group, the United Kingdom’s publicly-owned impact investor, Development Partners International (DPI), through its ADP III fund and the European Bank for Reconstruction and Development (EBRD).